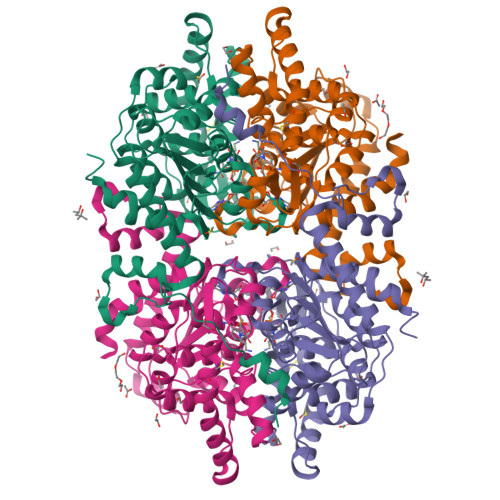
Half Way to Hypusine-Structural Basis for Substrate Recognition by Human Deoxyhypusine Synthase.
Wator, E., Wilk, P., Grudnik, P.(2020) Biomolecules 10
- PubMed: 32235505
- DOI: https://doi.org/10.3390/biom10040522
- Primary Citation of Related Structures:
6XXH, 6XXI, 6XXJ, 6XXK, 6XXL, 6XXM - PubMed Abstract:
Deoxyhypusine synthase (DHS) is a transferase enabling the formation of deoxyhypusine, which is the first, rate-limiting step of a unique post-translational modification: hypusination. DHS catalyses the transfer of a 4-aminobutyl moiety of polyamine spermidine to a specific lysine of eukaryotic translation factor 5A (eIF5A) precursor in a nicotinamide adenine dinucleotide (NAD)-dependent manner. This modification occurs exclusively on one protein, eIF5A, and it is essential for cell proliferation. Malfunctions of the hypusination pathway, including those caused by mutations within the DHS encoding gene, are associated with conditions such as cancer or neurodegeneration. Here, we present a series of high-resolution crystal structures of human DHS. Structures were determined as the apoprotein, as well as ligand-bound states at high-resolutions ranging from 1.41 to 1.69 Å. By solving DHS in complex with its natural substrate spermidine (SPD), we identified the mode of substrate recognition. We also observed that other polyamines, namely spermine (SPM) and putrescine, bind DHS in a similar manner as SPD. Moreover, we performed activity assays showing that SPM could to some extent serve as an alternative DHS substrate. In contrast to previous studies, we demonstrate that no conformational changes occur in the DHS structure upon spermidine-binding. By combining mutagenesis and a light-scattering approach, we show that a conserved "ball-and-chain" motif is indispensable to assembling a functional DHS tetramer. Our study substantially advances our knowledge of the substrate recognition mechanism by DHS and may aid the design of pharmacological compounds for potential applications in cancer therapy.
Organizational Affiliation:
Malopolska Centre of Biotechnology, Jagiellonian University, ul. Gronostajowa 7a, 30-387 Krakow, Poland.